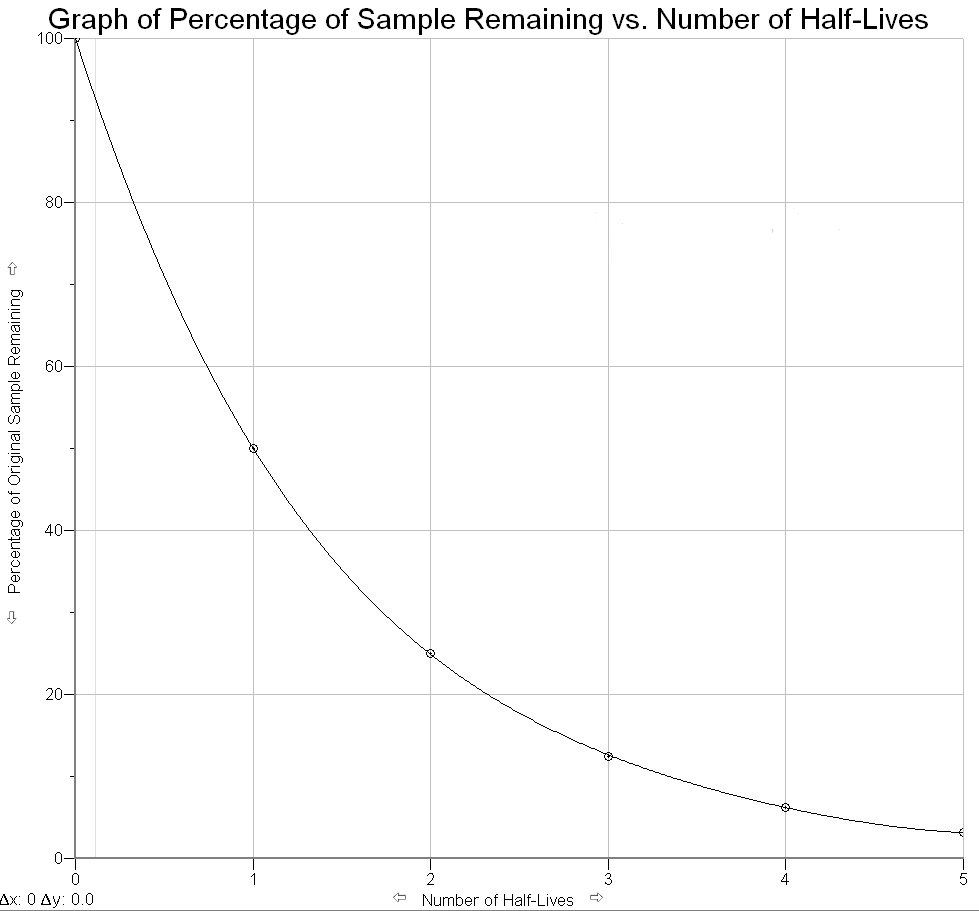
As mentioned previously, physicists measure the rate of radioactive decay by measuring the time it takes for half of a sample of an element to decay into another element. This time is known as an element’s half-life.
The half-life of uranium-238 (U-238), for example, is 4.5 billion years. This means that if we started with a sample of 100 grams of U-238 today, then after 4.5 billion years only 50 grams of the original sample would remain; 50 grams would have decayed into another element. After the next 4.5 billion years (or a total of 9.0 billion years from today) then half of the remaining sample would have decayed leaving only 25 grams of the original material (i.e. 1/2 × 1/2 = 1/4 of the original), and so on.
The ratio of the parent isotope to the daughter isotope indicates how much time has passed since the original sample was formed. The greater the proportion of the daughter product to the parent element, the older the sample is. This method of dating is known as radiometric dating and is the most common method used for determining the absolute age of rocks.
A number of radioactive isotopes are quite useful in dating rocks.
| Parent Isotope | Daughter Isotope | Half-Life of Parent (billions of years, i.e. × 109 years) |
| Uranium-238 | Lead-206 | 4.5 |
| Uranium-235 | Lead-207 | 0.7 |
| Thorium-232 | Lead-208 | 14 |
| Rubidium-87 | Strontium-87 | 48.8 |
| Potassium-40 | Argon-40 | 1.3 |
Radiometric dating does not work on sedimentary rocks since sedimentary rocks are formed from preexisting rocks. Radiometric dating works best on igneous rocks.